Imagine facing a stressful situation – maybe a thunderstorm quickly approaching or a surprise encounter on a hiking trail. Your body gears up, heart racing, senses heightened. Part of that healthy stress response often includes a subtle rise in core body temperature, preparing you for action.
For individuals with spinal cord injury (SCI), this natural response can be dramatically altered. SCI severely impacts the autonomic nervous system, which controls involuntary body functions like heart rate, blood pressure, and digestion. This disruption can lead to serious conditions like autonomic dysreflexia, where the body overreacts dangerously to stimuli.
Despite these widespread autonomic issues, how SCI affects the body's ability to control temperature, especially during stress, has been largely overlooked. Our latest preprint addresses this gap, revealing in rats how SCI affects crucial temperature-related stress responses.
Female and male rats were implanted with a small transmitter that measured activity and core temperature. Two weeks later, rats received T8 contusion SCI or sham laminectomy surgery.
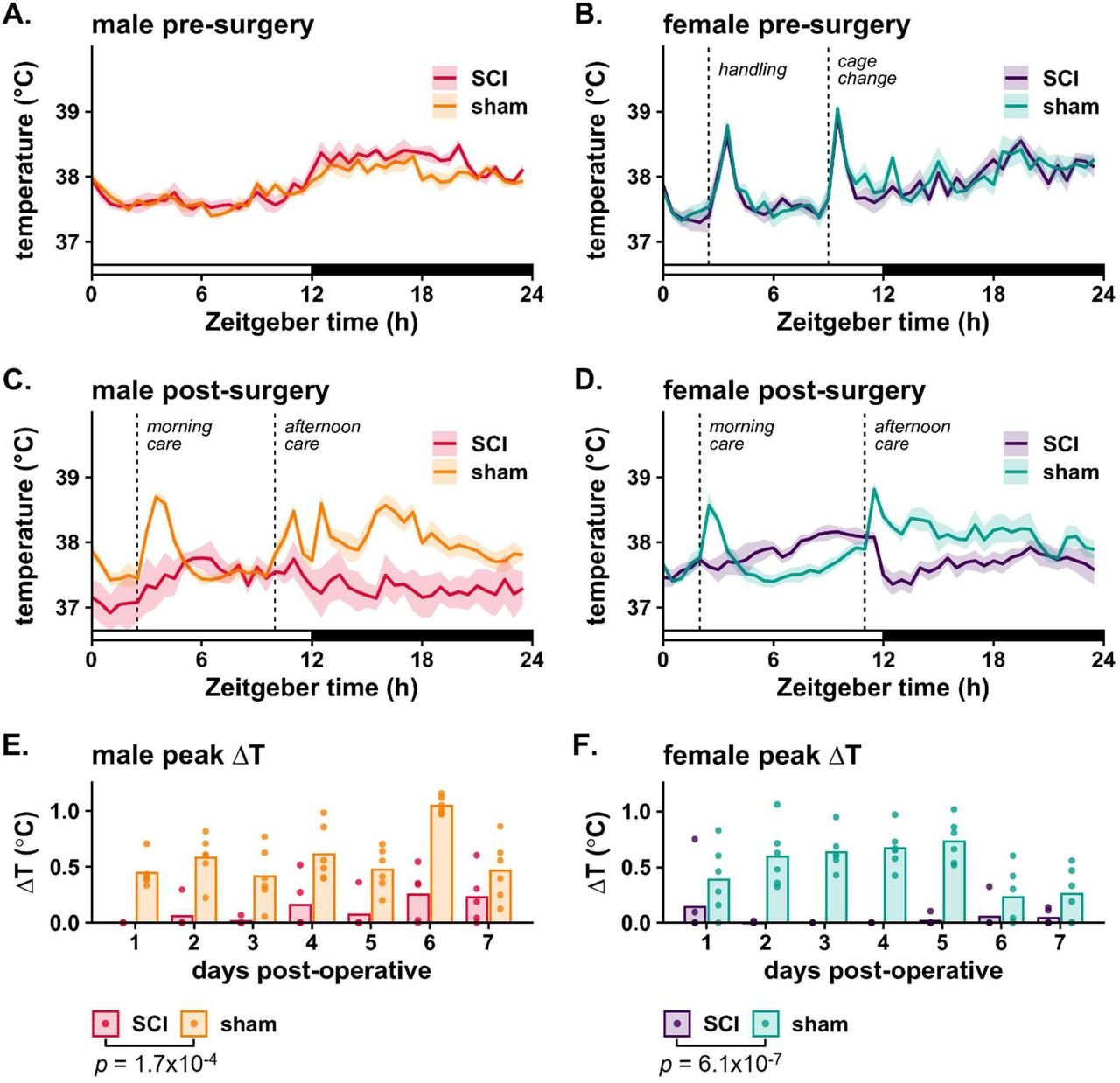
Next, we assessed body temperature across the day in rats prior to and after SCI (or sham) surgery (Fig. 1). Prior to surgery, both male and female rats exhibit expected daily rhythms in core temperature, with higher temperatures during the active (dark) phase. We noted that handling elicited a stress response, including increased activity and body temperature. In sham-surgery rats, this stress-induced hyperthermia is maintained immediately after surgery. In contrast, SCI rats lack stress-elicited hyperthermia in the acute phase after SCI. This SCI-driven loss of stress-induced hyperthermia occurs in both female and male rats.
Figure 1. The stress-induced hyperthermic response is abolished acutely after SCI.
(A,B) Average core temperature (± SEM) for male (A) and female (B) rats is shown at 1-2 weeks pre-surgery. The x-axis represents Zeitgeber Time (ZT), with ZT0 denoting lights on and ZT12 lights off. Handling/care events are annotated with vertical dashed lines. (C,D) Sham and SCI temperatures at 2 dpo – whereas sham rats show typical stress-induced hyperthermia, SCI rats lose this hyperthermic response. (E,F) Temperature peaks above background (ΔT) for sham and SCI rats for 7 dpo, with bars representing group means and points marking individual values. SCI disrupts stress-induced hyperthermia across the acute post-injury period. P-values, indicating significant main effects of surgery, were determined via mixed-effects model.
Remarkably, this temperature control deficit was not permanent (Fig. 2). Over several weeks post-injury, both male and female rats with SCI gradually regained their ability to mount a stress-induced hyperthermia response. Interestingly, females with SCI recovered their stress responses slightly faster than males.
Figure 2. Stress-induced hyperthermia is abolished over acute-to-subacute times after incomplete SCI, and is regained by chronic post-SCI times.
(A,B) Heatmaps display ΔT (temperature peaks above background) for individual male (A) and female (B) rats across 7 weeks post-surgery. The first care or interaction event of the day is shown. Cage changes are marked with “v”. Solid lines indicate twice-daily care (1–17 dpo for males and 1–14 for females), while dotted lines mark once-daily care (27 dpo for males and 28 dpo for females). Days with no care events or missing data are shaded in grey. (C,D) Modeled recovery curves for the stress-induced hyperthermic response in male and female animals are based on daily estimated marginal means derived from a mixed-effects model. Lines represent the modeled estimates ± standard error. Vertical lines indicate the estimated stress-hyperthermic recovery dates, defined as the first day when SCI stress-induced hyperthermia responses were statistically indistinguishable from sham responses (male SCI vs. male sham: 27 dpo; female SCI vs. female sham: 23 dpo) (p > 0.05). (E,F) Average core temperatures (± SEM) are shown for sham/SCI males and females on the estimated stress-induced hyperthermia recovery date (left panel, E and F) and during the final cage change at 7 weeks post-surgery (right panel). Room entries for cage changes are annotated with vertical dashed lines.
Our findings reveal a significant, and transient, disruption of temperature regulation and stress-induced hyperthermia following T8 SCI. This research opens new opportunities for understanding the broader impact of SCI on the autonomic nervous system. Ultimately, by uncovering and addressing these critical physiological deficits, we aim to help develop interventions that could improve the quality of life for individuals living with SCI.
This exciting work includes contributions by John Aldrich, Kalina Dusenbery, Linda Watkins, and Andrew Gaudet. We appreciate support provided by University of Texas at Austin start-up funds, the Wings for Life Foundation (Watkins/Gaudet), and NIH NINDS R01NS131806 (Gaudet).